
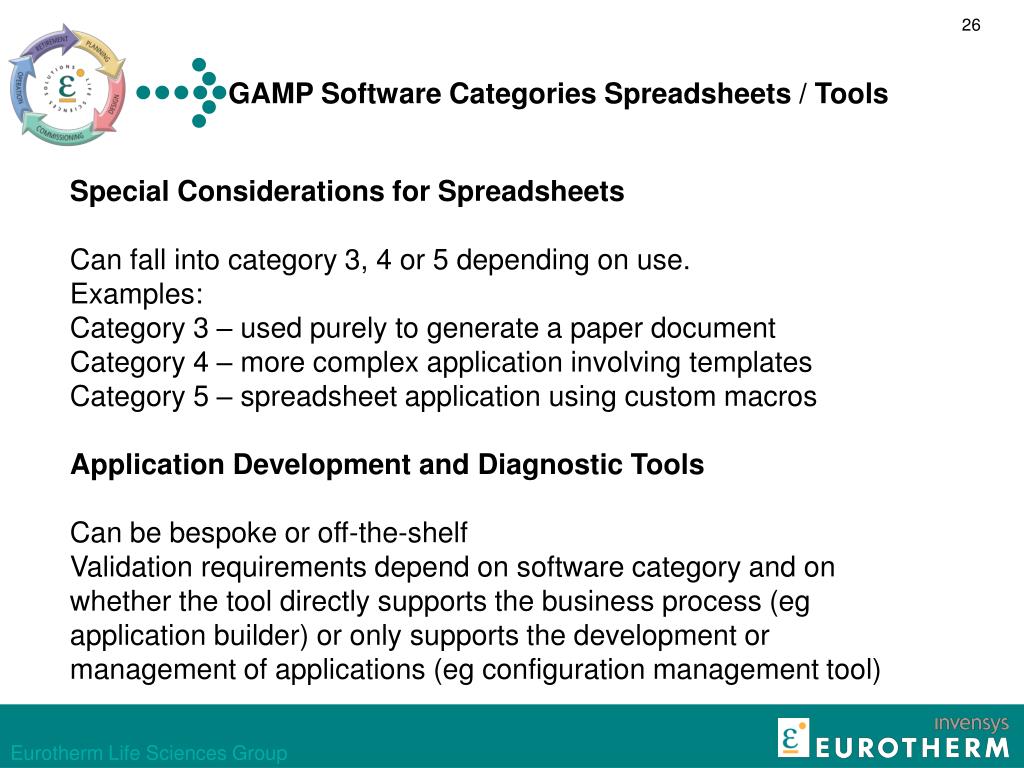

It is, therefore, not mandatory to follow this methodology. The strategies defined in GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems are guidelines, not regulations. If the test passes, the executed test script serves as documented evidence that the associated requirements and specifications were met. The test scripts are traced to the requirements and specifications they verify. This is accomplished by using the requirements and specifications as an objective standard to which the system is tested. The aim of conducting verifications is to demonstrate that the system functions as intended. As a result, the extent of the testing performed would also be reduced. However, functional and configuration specifications are not required when using commercial off-the-shelf software (Category 3). For example, for a configured product (Category 4), requirements, functional and configuration testing is conducted to verify the requirements, functional and configuration specifications. The types of specifications associated with a system are tied to its degree of complexity.
Gamp 5 software categories verification#
The V-model juxtaposes the specifications produced for a system to the testing performed as part of the verification process. GAMP®5’s approach can be summed up by the V-model diagram. Categorizing the system helps guide the writing of system documentation (including specifications and test scripts and everything in between). In a nutshell, GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems provides a framework for the risk-based approach to computer system validation where a system is evaluated and assigned to a predefined category based on its intended use and complexity. This document is published by an industry trade group called the International Society for Pharmaceutical Engineering (ISPE) based on input from pharmaceutical industry professionals. Usually, when one hears the terms GAMP®5, it is in reference to a guidance document entitled GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems. GAMP stands for Good Automated Manufacturing Practice.
Gamp 5 software categories how to#
You may be asking yourself: “What’s with all the hype? Is this a subject I should learn more about?” In this article, I’ll attempt to answer these questions by providing a brief overview of GAMP®5, by highlighting some of the benefits and by offering advice on how to apply its principles within your organization. Surely you have heard of it, but perhaps you are new to validation or you have limited experience in the validation of computerized systems. One of the most widely used terms in computer system validation jargon is GAMP®5. Add in buzzwords like gap analysis and risk management and sometimes it may seem like your company’s validation team is speaking a language all of their own. Just listen in on any discussion involving validation and you will likely be bombarded by them: URS, IQ, UAT, and GxP (we once put together a list, check it out here). One thing most validation professionals seem to agree on is that the industry uses a lot of acronyms.


 0 kommentar(er)
0 kommentar(er)
